Fusion: Why deuterium and tritium?
Physics Asked by user34771 on May 26, 2021
I would like to know why scientists try to use deuterium and tritium for fusion and not just the ordinary isotope of Hydrogen ${}^1H$?
3 Answers
The problem with attempting to fuse two protons is that there is no bound state $^2$He, for the rather obvious reason that there are no neutrons present to hold the two protons together. The fusion of two protons requires one of them to undergo beta plus decay while the two protons are close, and the probability of this is vanishingly small. It happens in the Sun because there are an awful lot of proton collisions in the Sun's core and even the tiny probability of fusion produces a sizable overall reaction rate.
By contrast fusing deuterium and tritium produces $^5$He, which does have a bound state, so this has a relatively large probability. The deuterium and tritium fuse to form $^5$He, and this then decays to $^4$He and a neutron with a half life of about $7 times 10^{-22}$ seconds.
See the related question: How much faster is the fusion we make on earth compared to the fusion that happens in the sun?
Answered by John Rennie on May 26, 2021
I agree with @john rennie, but I think it's worth noting that reaction rates for D-T are higher at a lower temperature as compared to D-D:
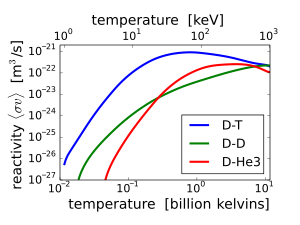
Higher temperatures present many difficult engineering challenges (even more so than the still high temperatures for D-T reactions).
Answered by Charles on May 26, 2021
The choice of fusion fuel is dictated by many factors:
- Its abundance or how easy/expensive it is to synthesise
- Its characteristics/behaviour in a reactor e.g. how does it move in an electromagnetic field? Is it easy to ionise?
- The types of fusion reactions that the fuel enables
For D and T:
- The fuel is relatively easy to synthesise (not very, but more practical than He3 for example)
- Using D and T fuel leads to reactions such as DD fusion and DT fusion. The latter releases a lot of energy, and is much more likely at achievable temperatures - hence the total power output is higher. The downside is that most of this power is carried by neutrons - which cannot be confined by electromagnetic fields. To achieve the same power output with other fuels, we would usually need an extremely hot and dense plasma, which would lead to the plasma collapsing due to Bremsstrahlung radiation. Likewise for fuels with other cross sections, one may find the fusion reaction is actually thermally unstable.
If we had much better confinement of the fuel, we would be able to use these alternative fuels at a lower temperature. Improving confinement is what most fusion science research is about :)
Answered by armoured-moose on May 26, 2021
Add your own answers!
Ask a Question
Get help from others!
Recent Questions
- How can I transform graph image into a tikzpicture LaTeX code?
- How Do I Get The Ifruit App Off Of Gta 5 / Grand Theft Auto 5
- Iv’e designed a space elevator using a series of lasers. do you know anybody i could submit the designs too that could manufacture the concept and put it to use
- Need help finding a book. Female OP protagonist, magic
- Why is the WWF pending games (“Your turn”) area replaced w/ a column of “Bonus & Reward”gift boxes?
Recent Answers
- Jon Church on Why fry rice before boiling?
- Lex on Does Google Analytics track 404 page responses as valid page views?
- Joshua Engel on Why fry rice before boiling?
- Peter Machado on Why fry rice before boiling?
- haakon.io on Why fry rice before boiling?